

岭南现代临床外科 ›› 2020, Vol. 20 ›› Issue (02): 146-150.DOI: 10.3969/j.issn.1009-976X.2020.02.003
摘要:
先天性巨结肠(hirschsprung"s disease,HD)是一种常见的肠神经系统发育障碍性疾病。因相关基因突变缺失或局部微环境的改变使神经母细胞在肠道迁移分化受阻,导致直肠结肠肠壁缺乏神经节细胞,病变肠管丧失蠕动功能而持续痉挛收缩,肠内容物排出受阻,近端肠管代偿性肥厚扩张。目前,HD以手术治疗为主,即切除无神经节细胞段肠管后再行肠吻合术。随着手术技术的不断进步,特别是近年来腹腔镜技术的发展,大大减少了手术创伤,明显改善患儿术后的生活质量,但便秘复发、失禁(污粪)、肛门狭窄以及小肠结肠炎等术后并发症仍有发生[1]。对全结肠型巨结肠或者巨结肠类源病患者,国内外仍无理想的治疗措施。
细胞移植是近年来HD治疗的研究热点之一,在2016年发布的肠神经疾病治疗指南中指出,HD是干细胞移植治疗的目标疾病[2]。尽管现有研究已成功的从废弃肠道组织中分离培养出肠神经干细胞[3],然而,从患儿肠道内获取肠神经干细胞具有较大的创伤性,肠神经干细胞的数量也非常有限,不能满足细胞移植治疗需要。因此,有必要寻找一种来源丰富的种子细胞。脂肪干细胞(adipose-derived stem cells,ADSCs)来源广泛,取材损伤小,其成神经分化、成骨分化、成软骨分化和成脂分化等多向分化潜能早已得到验证[4,5]。最近的实验研究发现,ADSCs在局部微环境中分泌各种神经营养因子,如 BDNF、PDGF-AB、VEGF、HGF、TGF-β、IGF-1、b-FGF,从而起到保护现存神经细胞功能、促进周围神经再生和降低局部组织炎症反应的作用,可应用于修复中枢神经系统损伤、脊髓神经损伤、神经性耳聋和坐骨神经损伤[7-12]。因此,ADSCs可能是治疗先天性巨结肠肠神经细胞缺失的理想种子细胞。目前国内外尚未见应用ADSCs转分化成肠神经干细胞的相关报道。本研究探讨应用小分子SB431542、noggin和LDN1931897将大鼠ADSCs转分化成肠神经样干细胞(enteric neural-like stem cells,ENLSC)的可行性,希望能够为HD的细胞治疗提供一种潜在的种子细胞来源。
1.1.1 动物 4周SD大鼠18只,体重约100 g,随机分成实验组及对照组,每组9只。E15d的SD孕鼠9只。购自中山大学医学院实验动物中心。
1.1.2 主要试剂 低糖DMEM培养基(Gibco公司,美国),DMEM/F12培养基(Gibco公司,美国),胎牛血清(Hyclone公司,澳大利亚),血清替代物(knock out serum replacement,KOSR)( Gibco 公司,美国),兔抗大鼠Sox2,Nestin抗体(Santa Cruz公司,美国),SB431542(BioVision公司,美国),noggin(PeproTech公 司 ,美 国),LDN1931897(Adooq Bioscience,美国),B27(Gibco公司,美国),N2(Gibco公司,美国),表皮生长因子(epidermal growth factor,EGF)(santa,美国)和碱性成纤维细胞生长因子(basic fibroblast growth factor,bFGF)。
1.2.1 ADSCs的分离培养鉴定参考本研究组前期文献方法进行[4,5]。取SD大鼠腹股沟脂肪组织,0.1%Ⅰ型胶原酶消化计数,以5×104有核细胞/cm2细胞密度接种,DMEM+10%FBS培养液传代培养至第三代。
1.2.2 ADSCs转分化实验 将第三代ADSCs接种在DMEM/F12培养基中,添加3%KOSR,1%Glutamax,1%非必需氨基酸和4 ng/mL bFGF,10 μM SB431542,100 ng/mL noggin 和 0.5 μM LDN 193289,培养 7天。培养基中添加2%B27,1%N2,20 ng/mL EGF,20 ng/mL bFGF和胎肠培养液(培养液配制参照文献[6]),分别培养7天和14天。对照组为未诱导ADSCs。
1.2.3 肠神经样干细胞的鉴定 ①形态学鉴定:倒置显微镜下观察细胞生长形态;②细胞增殖生长情况鉴定:将转分化培养14天的ENLSC消化成单个细胞进行CCK-8试验,用酶标仪测定各孔在450 nm处的吸光度(OD值),检测ENLSC的生长情况;③免疫荧光鉴定:检测Nestin、Sox2等神经干细胞标记物的表达情况。
1.2.4 统计学方法 应用统计学软件SPSS 16.0进行统计学分析。两组数据比较应用配对t检验。P<0.05为差异有统计学意义。
ADSCs向ENLSC转分化过程中细胞形态变化过程见图1。ADSCs在神经培养基中转分化悬浮培养7天,转分化的ADSCs表现出神经干细胞的行为特性,形成许多神经球样的细胞团块。将神经球样细胞团转入肠神经培养基中继续诱导培养7天,可见细胞再次聚集成团生长,细胞团内细胞变成圆形或者椭圆形。在诱导14天后形成类似于神经球样的细胞聚合物,有长长的突起“锚”在培养皿底面,与周围贴壁细胞相互形成网络连接。对照组仅见贴壁的梭形脂肪干细胞生长,未见细胞聚集成团。

图1 光镜下观察ENLSC的细胞形态变化
A:ADSCs在神经培养基中转分化悬浮培养7天,细胞聚集生长形成神经球样的细胞团块;B:转入肠神经培养基中培养7天的ADSCs呈聚集性生长,团块内细胞呈圆形或者椭圆形;C:肠神经培养基中培养14天的ADSCs形成神经球样细胞团,并见许多长长的突起“锚”在培养皿底面,与周围贴壁细胞相互形成网络连接;D:对照组ADSCs未见形态改变。(×40)
将转分化诱导培养14天的ENLSC和未诱导的ADSCs,利用CCK-8法测得ENLSC在1、2、3、4、5、6和7天等7个时间点的OD值,作出实验组和对照组的生长曲线。由图2可知,在转分化培养7天,ENLSC生长增殖趋势良好。与对照组比较,两组无明显差异(P=0.084)。说明经过转分化诱导后的ENLSC仍然能够保持良好的增殖分化能力。
对诱导转化14天和21天的ENLSC进行巢蛋白(Nestin)和Sox2免疫荧光染色,见图3。诱导转化第14天和第21天,荧光显微镜下可见ENLSC均呈现Nestin阳性表达。第21天Nestin阳性表达的细胞数量和强度均较第14天明显。对照组在第14天和第21天均未见Nestin阳性表达。诱导转化第14天和第21天,荧光显微镜下可见ENLSC均有Sox2阳性表达,细胞间可见细小的突触连接。对照组在第14天和第21天均未见Sox2阳性表达。
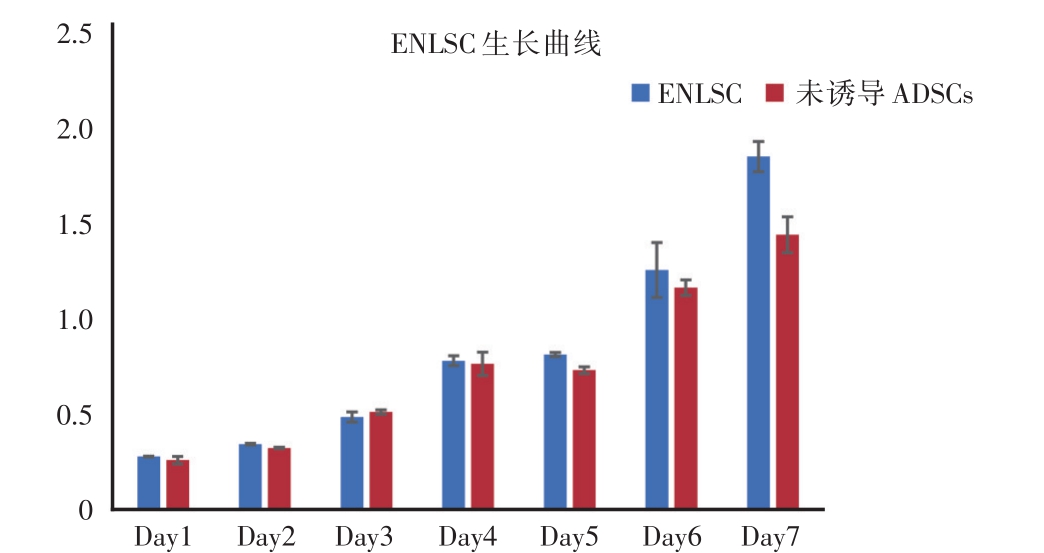
图2 CCK-8法测定ENLSC生长曲线
ENLSC组与未诱导ADSCs组无明显统计学差异(P=0.084)
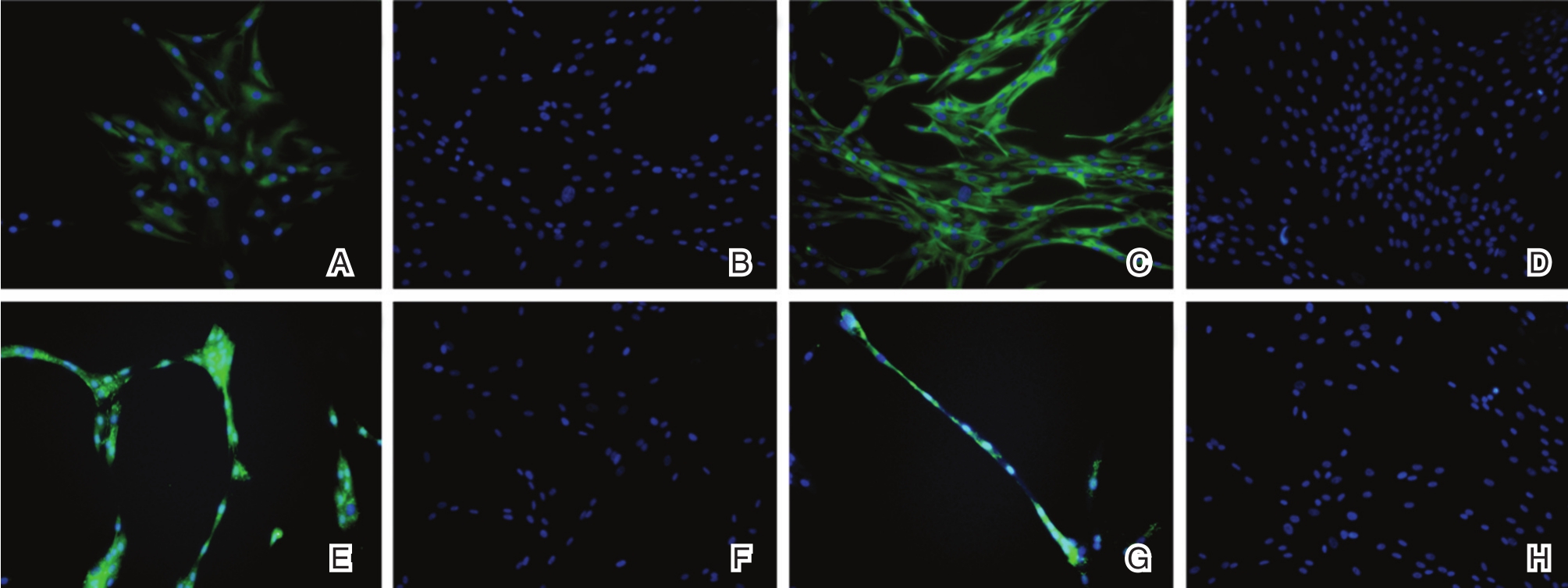
图3 免疫荧光染色检测肠神经干细胞特异性标志物
A:ADSCs转分化诱导14天,Nestin免疫荧光染色呈阳性表达;B:对照组ADSCs未见Nestin阳性表达;C:ADSCs转分化诱导21天,Nestin呈阳性表达,阳性表达细胞数量较第14天时增多增强;D:对照组ADSCs未见Nestin阳性表达;E:ADSCs转分化诱导14天,Sox2免疫荧光染色呈阳性表达;F:对照组ADSCs未见Sox2阳性表达;G:ADSCs转分化诱导21天,Sox2呈阳性表达,细胞间相互连接;H:对照组ADSCs未见Sox2阳性表达。(×200)
HD的病因主要是直结肠肠壁的神经节细胞缺失,细胞移植治疗在HD神经功能修复重建中具有巨大的应用前景。现有的研究主要是通过体外增殖诱导分化神经干细胞或者肠神经干细胞成肠神经细胞作为细胞移植的细胞来源。由于肠神经细胞是一种成熟终末分化细胞,其缺乏自我增殖分化能力,移植后只能暂时存活于被移植组织中,移植效果不理想。因此,具有自我增殖和分化能力的干细胞移植才是理想的治疗方式。
近年,有学者应用9种小分子将小鼠成纤维细胞直接转分化为小鼠NSCs,揭示了仅使用小分子产生人神经干细胞和神经元的可行性[13,14]。小分子还能促进人ADSCs分化成表达Sox1,Pax6和NF-H的神经元样细胞[15]。Park J等认为由于小分子激活体内特定的细胞转化通路机制产生靶细胞的方式与体内类似,使用小分子激活内源性神经基因来产生神经细胞更安全和更稳定[16]。我们的实验初步提示了ADSCs在小分子SB431542、noggin和LDN1931897的共作用下呈现出类似神经干细胞的行为特征。由原来的长梭形细胞逐渐转变成圆形或者椭圆形,最后形成许多神经球样的细胞团块。ENLSC阳性表达Nestin,一种中间丝蛋白,在哺乳动物神经前体细胞中高表达,是神经干细胞的特征性标志物,提示转分化细胞正处于干细胞阶段[14]。在体外扩增培养时,ENLSC所表现的增殖能力与未诱导的ADSCs无明显差异,说明ENLSC能保持良好的自我增殖能力。
虽然肠神经干细胞特异性标志物仍存在争议,但Heanue TA等在肠神经系统研究中发现,Sox2在肠神经系统前体细胞中表达,并认为可作为肠神经干细胞识别和筛选的新标志[17]。Sox2是脊椎动物早期发育中最早表达的神经系统特异性基因之一,同时在干细胞的维持中也起着关键作用,并常被作为一种多能性细胞谱系的分子标记。Sox2基因也广泛存在于神经祖细胞、神经干细胞和少数成熟神经元中,是神经功能决定因子和神经干细胞特性维持所必需的[18]。我们诱导转化第14天和第21天的ENLSC均稳定表达Sox2,提示转分化细胞具有肠神经干细胞相关标志物阳性表达。
综上所述,我们应用小分子SB431542、noggin和LDN1931897转分化获得的ENLSC能够表现出肠神经干细胞的部分特征,具有良好的增殖能力,可能能够为肠神经干细胞的细胞移植治疗提供一种潜在的种子细胞来源。然而,肠神经干细胞移植的最终目标是恢复移植肠道的功能,本研究仍有许多不足之处。比如,ENLSC是否能够进一步分化成功能性的肠神经细胞,是否能够定植存活在被移植肠管仍有待进一步的实验验证。
[1] Tang ST,Wang GB,Cao GQ,et al.10 years of experience with laparoscopic-assisted endorectal Soave pull-through procedure for Hirschsprung′s disease in China[J].J Laparoendosc Adv Surg Tech A,2012,22(3):280-284.
[2] Burns AJ,Goldstein AM,Newgreen DF,et al.White paper on guidelines concerning enteric nervous system stem cell therapy for enteric neuropathies[J].Dev Biol,2016,417(2):229-251.
[3] 胡书奇,岳雷,夏肇波,等.先天性巨结肠患儿肠神经干细胞培养的实验研究[J].中华小儿外科杂志,2015,36(11):846-850.
[4] 伍耀豪,崔磊,尹烁,等.猪脂肪干细胞体外多向分化潜能的实验研究[J].组织工程与重建外科杂志,2007,3(6):333-338.
[5] Cui L,Wu Y,Cen L,et al.Repair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid mesh [J].Biomaterials,2009,30(14):2683-2693.
[6] 吴晓娟,魏明发,柴成伟,等.大鼠骨髓间充质干细胞体外向肠神经细胞分化及胶质细胞源神经营养因子表达的变化[J].中华小儿外科杂志,2010,31(8):607-611.
[7] Zhao K,Li R,Gu C,et al.Intravenous Administration of Adipose-Derived Stem Cell Protein Extracts Improves Neurological Deficits in a Rat Model of Stroke[J].Stem Cells Int,2017,2017:2153629.
[8] Park SS,Lee YJ,Lee SH,et al.Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal Stem cells[J].Cytotherapy,2012,14(5):584-597.
[9] Orbay H,Uysal AC,Hyakusoku H,et al.Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps[J].J Plast Reconstr Aesthet Surg,2012,65(5):657-664.
[10] Jang S,Cho HH,Kim SH,et al.Transplantation of human adipose tissue-derived stem cells for repair of injured spiral ganglion neurons in deaf guinea pigs[J].Neural Regen Res,2016,11(6):994-1000.
[11] Marconi S,Castiglione G,Turano E,et al.Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush[J].Tissue Eng Part A,2012,18(11-12):1264-1272.
[12] Jeon D,Chu K,Lee ST,et al.Neuroprotective effect of a cellfree extract derived from human adipose stem cells in experimental stroke models[J].Neurobiol Dis,2013,54:414-420.
[13] Cao N,Huang Y,Zheng J,et al.Conversion of human fibroblasts into functional cardiomyocytes by small molecules[J].Science,2016,352(6290):1216-1220.
[14] Zhang M,Lin YH,Sun YJ,et al.Pharmacological Reprogramming of Fibroblasts into Neural Stem Cells by Signaling-Directed Transcriptional Activation[J].Cell Stem Cell,2016,18(5):653-667.
[15] Madhu V,Dighe AS,Cui Q,et al.Dual Inhibition of Activin/Nodal/TGF-β and A2018033 BMP Signaling Pathways by SB431542 and Dorsomorphin Induces Neuronal Differentiation of Human Adipose Derived Stem Cells[J].Stem Cells Int,2016,2016:1035374.
[16] Park J,Lee N,Lee J,et al.Small molecule-based lineage switch of human adipose-derived stem cells into neural stem cells and functional GABAergic neurons[J].Sci Rep,2017,7(1):10166.
[17] Heanue TA and Pachnis V.Prospective identification and isolation of enteric nervous system progenitors using Sox2[J].Stem Cells,2011,29(1):128-140.
[18] Ring KL,Tong LM,Balestra ME,et al.Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor[J].Cell Stem Cell,2012,11(1):100-109.
A preliminary study on the transdifferentiation of adipose-derived stem cells into enteric nerve-like stem cells
中图分类号: